 Loading... Please wait...
Loading... Please wait...- Home
- Anti Obesity
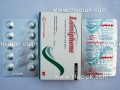
- Nobese Sibutramine HCL 15 mg by Getz Pharma 7 capsules / Strip
Product Description
Nobese of Getz Pharma is the first orally administered serotonin (5-hydroxytryptamine, 5-HT) and noradrenaline reuptake inhibitor (SNRI) used for the management of obesity. Nobese is chemically Sibutramine Hydrochloride.
How Nobese Works
Nobese has been shown to reduce body weight by dual actions: reduction in food intake through enhancement of satiety and increase of energy expenditure by induction thermogenesis. Nobese produces its therapeutic effects primarily by serotonin and noradrenaline reuptake inhibition.
Absorption
Nobese is rapidly absorbed from the Gartro Intestinal Tract following oral administration. Peak plasma concentration of the parent drugu appears after 1.2 hours and of its pharmacologically active metabolites M1 and M2, three to four hours, on average, atleast 77% of a single oral dose of Nobese is absorbed.
Distribution
Nobese and its metabolites M1 and M2 are extensively bound (97% and 94%) respectively. To human plasma protein. It is extensively distributed to the renal and hepatic tissues.
Metabolism
Nobese undergoes extensive first-pass metabolism. Nobese is principally metabolized by the cytochrome P450 isoenzyme CYP3A4 to two demethyled active metabolites which are secondary and primary amine metabolites. These active metabolites are further metabolized by hydroxylation and conjugation to inactive M5 and M6 metabolites.
Illumination
The primary route of excretion for the active metabolites is hepatic metabolism and for inactive metabolites is renal excretion. Plasma illumination half life is 14-16 hours.
Hepatic Insufficiency
In patients with moderate hepatic impairment receiving a single 15 mg oral dose of Nobese, the combined AUCs of M1 and M2 increased by 24% which does not warrant dose adjustment.
Renal Insufficiency
Illumination of inactive hydroxyl metabolites, which are renally excreted, may be affected in this population.
Indications
- Nobese is indicated for management of obesity including weight loss and weight maintenance When used in conjunction with a reduce calorie diet.
- In patients with an initial body mass index <30kg/m or <27kg/m in the presence of oter risk factors (hypertension, disbetes, dislipidemia).
Dosage
The recommended initial oral dose of nobese is 10 mg capsule once daily with or without food. If there is inadequate weight loss (i.e. <20kg) the dose may be increased to 15 mg provided 10 mg was well tolerated. The 5 mg dose should be reserved for patients who do not tolerate the 10 mg dose.
Nobese treatment should not be given for longer than 1 year. Maximum weight loss (5%-10% of initial body weight) is usually achieved within six months. Nobese must be discontinued in patients who have not responded adequately, i.e. whose weight loss stabilizes at less than 5% of their initial body weight or whose weight loss within three months after starting therapy has been less than 5% of their initial body weight. Nobese should not be continued in patients who regain 3kg or more after previously achieved weight loss.
Adverse Reaction
Most of the adverse reactions of Nobese occur at the start of the treatment (1st 4 weeks). There severity and frequency diminishes over time. The frequently observed events are;
- Dry mouth
- Headache
- Insomnia
- Constipation
- Diarrhea
- Back pain
- Increased apatite
- Dizziness
- Influenza like symptoms
- Rhinitis
Less Frequent Adverse Reactions with Nobese
- Dyspepsia
- Nausea
- Dysmenorrhea
- Increased sweating and thirst
- Oedema
- Parathesia
- Taste perversion
- Drossiness
- Skin rashes
- Palpitation
- Vasodilatation
- Anxiety
- Nervousness
- Depression
Rare Adverse Reactions with Nobese
- Abnormal bleeding including Henoch-Schonlein purpura
- Thrombocytopenia
- Acute interstitial nephritis
- Glomerulo nephritis
- Emotional lability
- Seizures
- Blurred vision
- Gall stone formation
- Reversible increase in liver enzymes
Contra Indications
Nobese is contraindicated in patients;
- With known hypersensitivity to sibutramine hcl or any of the components of the product.
- Receiving monoamine oxidase inhibitors (MAOIs) and serotonin reuptake inhibitors (SSRIs) resulting in inverted “serotonin syndrome”.
- With organic causes of obesity.
- Who have major history of eating disorders such as anorexia nervosa or bulimia nervosa.
- With gillus de la tourette’s syndrome.
- With a history of coronary artery disease, congestive heart failure, tachycardia, peripheral arterial occlusive disease, arrhythmia or cerebrovascular disease (stroke or ITA).
- With inadequately controlled hypertension (>145/90mmHg).
- With hyperthyroidism.
- With severe liver impairment.
- With benign prostatic hyperplasia with urinary retention.
- With pheaochromocytoma.
- With narrow angle glaucoma.
- With the history of drug, medication or alcohol abuse.
- Who are pregnant or breast feeding.
- Above 55 years.
Nobese is also contraindicated in children and young adults up to age of 18 years.
Precautions
- Nobese causes increase in blood pressure in some patients therefore cardiovascular parameters should be monitored every two weeks in the first three months of the treatment, and thereafter regularly at maximum intervals of three months.
- Caution should be taken when Nobese is given to the patients with a history of motor or verbal tics.
Drug Interactions
- Co-administration of Nobese is contraindicated with MAOIs, therefore there should be two weeks interval between discontinuation of MAOIs and initiation of treatment with Nobese and vice versa.
- Concomitant use of Nobese with drugs like decongestants, cough, cold and allergy medications (Phenylpropanolamine, ephedrine or pseudophedrine) rases blood pressure or heart rate and therefore should be used with caution.
- Co-administration of cytochrome P450 (3A4) enzyme inhibitors (ketoconazole, erythromycin and cimetindine) increases plasma concentration of Nobese. Caution should be exercised on concomitant administration of Nobese with other enzyme inhibitors.
Customers Who Viewed This Product Also Viewed
-
$3.99

-
$3.95

-
$13.50

-
$6.85