 Loading... Please wait...
Loading... Please wait...- Home
- Oral Steroids
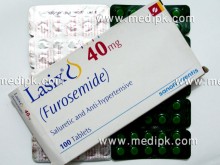
- Lasix 40 mg by sanofiaventis 100 tablets Pack / Strip 50 tabs
Lasix 40 mg by sanofiaventis 100 tablets Pack / Strip 50 tabs
Product Description
LASIX® 20 mg (tablets)
LASIX® 40 mg (tablets)
LASIX® 80 mg (tablets)
LASIX® 2 mL Injection
LASIX® Oral Solution
SCHEDULING STATUS:
S3
PROPRIETARY NAME
(and dosage form):
LASIX® 20 mg (tablets)
LASIX® 40 mg (tablets)
LASIX® 80 mg (tablets)
LASIX® 2 mL Injection
LASIX® Oral Solution
COMPOSITION:
| Tablet 20 mg | : | Each tablet contains 20 mg furosemide |
| Tablet 40 mg | : | Each tablet contains 40 mg furosemide |
| Tablet 80 mg | : | Each tablet contains 80 mg furosemide |
| Injection 2 mL | : | Each ampoule contains 20 mg furosemide |
| Oral Solution | : | Each 1 mL contains 10 mg furosemide Alcohol (94% m/m) 10% m/v Preservatives: Methylparaben 0,25% m/v Propylparaben 0,05% m/v |
PHARMACOLOGICAL CLASSIFICATION:
A 18.1: Diuretics
PHARMACOLOGICAL ACTION:
LASIX® inhibits the reabsorption of sodium and water predominantly in the ascending loop of Henle but also in the proximal tubule. It is often possible, in situations where other methods of treatment fail to induce diuresis, to increase the excretion of sodium and water with LASIX®, even when glomerular filtration rate is markedly impaired.
LASIX® lowers pathologically raised blood pressure, but does not affect normal levels.
With oral administration of LASIX®, the onset of action is rapid, usually within half an hour. Peak action is usually achieved after two hours, and the duration of action is 4 to 5 hours. With parenteral administration, the onset of action is even more rapid.
INDICATIONS:
Cardiac oedema: All forms of cardiac oedema in conjunction with adequate glycoside therapy.
Ascites due to cirrhosis of the liver, mechanical obstruction or cardiac failure.
Renal oedema in nephrotic syndrome.
Oedema occurring during the last three months of pregnancy-pre-eclamptic toxaemia and eclampsia.
As an adjunct in acute pulmonary oedema.
Cerebral oedema.
Hypertension of mild to moderate degree.
Barbiturate poisoning (using the principle of "forced diuresis").
Burns: to reduce local oedema and to prevent oliguria from progressing to complete anuria.
CONTRA-INDICATIONS:
Patients who are hypersensitive to furosemide or sulphonamides.
LASIX® is contra-indicated if increasing azotaemia and oliguria occur during treatment of severe progressive renal disease, anuria, hypokalaemia, hyponatraemia, hypovolaemia with or without hypotension. In hepatic coma and in states of electrolyte depletion, therapy with LASIX® should not be instituted until the basic condition is corrected or improved.
Furosemide should not be given to lactating women.
Furosemide should be administered during pregnancy only if strictly indicated, and then only for short periods of time.
DOSAGE AND DIRECTIONS FOR USE:
The usual dose of LASIX® is 20 mg to 80 mg per day given as a single dose preferably in the morning.
This dose may, however, be increased depending on the response of the patient. Six hours after a 40 mg dose, 80 mg may be administered and, if necessary, after another six hours, 120 mg. After the oedema is controlled, maintenance therapy is continued at 20 mg to 40 mg daily. Daily doses exceeding 120 mg should preferably be distributed over two to three individual doses.
For the treatment of hypertension of mild or moderate degree, a daily dosage of 40 to 80 mg is taken orally. In combination with other hypotensive drugs, lower doses will often suffice.
Intravenous or intramuscular administration of LASIX® is indicated in all cases where intestinal absorption is impaired or prompt diuresis required. The rapid and powerful effect produced by intravenous injection may result in a transitory fall in plasma volume.
Pulmonary oedema: Initial dose 40 mg intravenously. If necessary, the injection may be repeated after approximately 20 minutes.
Forceddiuresis (eg. management of barbiturate poisoning):
20 mg to 40 mg LASIX® is given in addition to infusion of electrolyte solution. Further treatment depends on the elimination of urine and must include substitution of the fluid and electrolyte losses. In poisoning with acid or basic substances the elimination rate can be further increased by alkalisation or acidification of the urine, respectively.
Infants and Children under 15 years:
Children generally receive an oral dose of 2 mg/kg body mass per day in divided doses. This may be titrated to a maximum of 6 mg/kg.
Parenteral administration (if necessary, continuous drip infusion) is indicated only in life-threatening conditions.
In this case, infants/children receive parenteral doses of 1 mg/kg body mass per day up to a maximum of 20 mg per day.
Administration:
Intravenous or intramuscular administration of LASIX® is indicated in all cases where intestinal absorption is impaired or rapid fluid elimination is necessary. Intravenously, LASIX® should be injected slowly. The rate of injection of 4 mg per minute should not be exceeded. During long-term treatment, serum creatinine and urea and also electrolytes, in particular potassium, calcium, chloride and bicarbonate, should be regularly checked.
Furosemide, being an anthranilic acid derivative, dissolves in alkaline media with salt formation. The solution for parenteral application contains the sodium salt of the carboxylic acid without a solubilizer. The solution has a pH of about 9 but no buffer capacity, which means that the drug may precipitate at pH values below 7. If the ready-to-use solution has a pH ranging from weakly alkaline to neutral, the mixture may be used for up to 24 hours.
LASIX® must not be mixed with other drugs in the same injection syringe.
The duration of treatment is at the physician's discretion.
SIDE-EFFECTS AND SPECIAL PRECAUTIONS:
Although administration of LASIX® only rarely leads to hypokalaemia, a potassium rich diet (lean meat, potatoes, bananas, tomatoes, cauliflower, spinach, dried fruit, etc.) is always advisable. Treatment with potassium-containing or potassium sparing preparations may be indicated.
As with other diuretics, electrolyte and water balance may be disturbed as a result of diuresis after prolonged therapy.
Mainly at the start of the treatment, excessive diuresis, particularly in elderly patients, may give rise to circulatory disturbances, such as a feeling of pressure in the head, vertigo or visual impairment: in extreme cases hypovolaemia, dehydration, dryness of mouth, circulatory collapse and blood coagulation disorders may also occur. However, with individualized dosage, acute haemodynamic reactions are generally not to be expected, although diuresis sets in rapidly.
LASIX® may cause potassium depletion, especially in cases of low potassium diet, vomiting or chronic diarrhoea. In addition, diseases such as cirrhosis of the liver may cause a predisposition to potassium deficiency states. Appropriate surveillance and replacement therapy are necessary in such cases.
If salt intake is restricted too much, sodium deficiency may produce a fall in blood pressure, calf muscle cramps, anorexia, weakness, dizziness, drowsiness, vomiting and confusional states.
The serum calcium level may be reduced under LASIX® therapy: in very rare cases tetany has been observed. In premature infants calcium salts may be deposited in the renal tissue (nephrocalcinosis). When administered to premature infants with respiratory distress syndrome in the first few weeks after birth, diuretic treatment with furosemide may accentuate the risk of a patent ductus arteriosus.
Gastro-intestinal disorders (e.g. nausea, vomiting, diarrhoea) or allergic reactions (e.g. rashes, vasculitis, interstitial nephritis, photosensitivity, vesicular cutaneous eruptions, fever and shock) and changes of the blood picture (leukopenia, agranulocytosis, haemolytic anaemia, thrombocytopenia) may occasionally be observed.
Anaphylactic shock, though rare, is an acute life-threatening reaction, and may occur only during parenteral administration.
Anaphylactic shock must be treated with the usual agents i.e. adrenaline, corticosteroids and antihistamines.
Symptoms of obstructed micturition (e.g. in hydronephrosis, prostatic hypertrophy, uretherostenosis) may become manifest or aggravated under the action of diuretics.
In common with other diuretics, treatment with LASIX® may induce a transient rise in serum creatinine and urea levels.
It should be remembered that an increase in uric acid concentration in the blood may precipitate attacks of gout in predisposed patients.
Serum cholesterol and triglyceride levels may increase under LASIX® treatment but will usually return to normal under long-term treatment, within six months.
In rare cases manifest diabetes mellitus may be aggravated by furosemide treatment and latent diabetes may become manifest. Isolated cases of acute pancreatitis have been reported in which the treatment with saluretics over several weeks was considered a causal factor, including also a few cases following therapy with furosemide.
Disorders of hearing after furosemide are rare and in most cases reversible. This possibility should be borne in mind, especially if furosemide is injected too rapidly and in particular in patients with renal insufficiency (see Administration).
Pre-existing metabolic alkalosis may be aggravated by furosemide treatment (e.g. in decompensated cirrhosis of the liver).
In individual cases the ability to drive or to operate machinery may be impaired, especially at the commencement of treatment or when changing over from other medicines or when alcohol is consumed during LASIX® therapy.
Interactions:
When a cardiac glycoside is administered concurrently it should be remembered that potassium deficiency increases the sensitivity of the myocardium to digitalis. In case of glucocorticoid medication or abuse of laxatives the risk of increased potassium loss should be borne in mind.
LASIX® may potentiate the nephrotoxic effects of certain antibiotics (e.g. aminoglycosides). Therefore, LASIX® should be used with caution in patients with antibiotic-induced renal impairment.
It should be borne in mind that the ototoxicity of aminoglycoside antibiotics (e.g. kanamycin, gentamicin, tobramycin) may be potentiated when LASIX® is used concurrently. The hearing defects that result may be irreversible. Therefore this drug combination should be restricted to vital indications. As the concomitant administration of cisplatin and parenteral LASIX® carries the risk of inducing hearing defects, the two medicines should not be used simultaneously.
Sometimes LASIX® may diminish the potency of other medicines (e.g. the effect of anti-diabetics and pressor amines) or potentiates their effect (e.g. in the case of salicylates, theophylline, lithium and curaremimetic muscle relaxants).
The action of other hypotensive medicines may be potentiated by LASIX®. Especially in combination with ACE-inhibitors a marked fall in blood pressure may be seen. Non-steroidal anti-inflammatory agents (e.g. indomethacin, acetylsalicylic acid) may antagonise the action of LASIX® and may cause renal failure in case of pre-existing hypovolaemia.
Concurrent administration of furosemide and sucralfate should be avoided as sucralfate reduces the absorption of furosemide and hence weakens its effect.
KNOWN SYMPTOMS OF OVERDOSAGE AND PARTICULARS OF ITS TREATMENT:
After the ingestion of an overdose there is some danger of dehydration and electrolyte depletion due to excessive diuresis.
The guiding principle of treatment is water and electrolyte replacement in accordance with urine output (with monitoring of carbohydrate metabolism if necessary). If difficulty in micturition is proved or suspected, as in cases of prostatic hypertrophy or impairment of consciousness, care must be taken to ensure a free outflow of urine from the bladder.
IDENTIFICATION:
| Tablet 20 mg: | A white scored tablet with the company logo on the one side, and coded DLF on the other side. Diameter: 6 mm. |
| Tablet 40 mg: | A white, scored tablet with the company logo on the one side, and coded DLI on the other side. Diameter: 8 mm. |
| Tablet 80 mg: | A white, flat tablet, 8-faceted, with a score line and "Lasix 80" imprinted on one side and the company logo on the other side. |
| Injection 2 mL: | A clear, colourless solution in an amber glass ampoule. |
| Oral Solution: | A clear orange-yellow solution with an odour of orange in a 100 mL amber glass bottle with a screw cap. |
PRESENTATION:
| Tablets 20 mg | : | Amber glass bottles of 30 tablets |
| Tablets 40 mg | : | Amber glass bottles of 30 and 250 tablets |
| Tablets 80 mg | : | Amber glass bottles of 30 and 100 tablets |
| Injections 2 mL | : | Cartons of 5 amber glass ampoules |
| Oral Solution | : | 100 mL amber glass bottles |
STORAGE INSTRUCTIONS:
| Tablets | : | Store below 25°C and protect from light. Exposure to light may produce a yellowish discolouration. |
| Injection | : | Store below 25°C and protect from light. |
| Oral Solution | : | Store between 2°C and 8°C in a refrigerator. Do not freeze. Protect from light. Dispense in light resistant containers. Once opened, the contents of the bottle remains stable for a period of 60 days under refrigerated conditions. |
KEEP OUT OF REACH OF CHILDREN.

Product Reviews
-
Good:)

Posted by rose -j on 1st Jul 2012
Continually, when men and women manage emempylont seeks they can deal with emempylont paying around my topic also known as job opportunities in my specific geographic location. There is nothing other in that. In addition, across frustrating economical occasions when women and men is required to be modest additional potent by getting a task search engine. Within the event you look at it, the crucial reason that people young and old want to know a activity associated with neighborhood is about efficiency. Whatever effortless to have emplyment within the vicinity of residence hold the way it drops the hassles, duress, and / or expense on carrying. Another reason why that males attempt occupations hiring into my field is genuinely because seriously is invaluable staying near home inside the event that numerous vital internal send out appears. Nevertheless this is to speak about surely nothing of their advantages engaging in case you might have school children.
Customers Who Viewed This Product Also Viewed
-
$3.99

-
$3.50

-
$4.50

-
$2.50